DPPH: Data Protection in Personalized Health
Personalized Medicine, Personalized Health Research Project funded by the Strategic Focus Area Personalized Health and Related Technologies (PHRT) of the ETH Board between April 2018 and December 2021.
Jacques Fellay
Head of Fellay Group, School of Life Science, EPFL & Head of Precision Medicine Unit,
CHUV
Bryan Ford
Head of DEDIS lab, School of I&C,
EPFL
Jean-Pierre Hubaux
Head of LDS, School of I&C,
EPFL (coordinator)
Dimitar Jetchev
Head of GR-JET, School of Basic Sciences,
EPFL
Effy Vayena
Head of Health Ethics and Policy Lab, Dept of Health Sciences and Technology,
ETH
Olivier Verscheure
Executive Director,
SDSC
This is the project abstract as envisioned at the time of its creation:
P4 (Predictive, Preventive, Personalized and Participatory) medicine is called to revolutionize healthcare by providing better diagnoses and targeted preventive and therapeutic measures. However, to accelerate its adoption and maximize its potential, clinical and research data on large numbers of individuals must be efficiently shared between all stakeholders. The privacy risks stemming from disclosing medical data raise serious concerns, and have become a barrier that can hold back the advances in P4 medicine if effective privacy-preserving technologies are not adopted to enable privacy-conscious medical data sharing. The evolution of the regulation towards further guarantees (e.g., HIPAA in USA and the new GDPR in EU) reflects this urgent need.
Pairing privacy-conscious data sharing with recent advances in the field of *omics and, in particular, in high-throughput sequencing technology, leads to an explosive growth in the amounts of available data; this big data scale can usually not be handled with current hospital computing facilities, hence the need for elastic computing resources that can cope with huge amounts of data in a secure and privacy-aware infrastructure, supporting data processing and sharing.
This project seeks to address the main scalability, privacy, security and ethical challenges of data sharing for enabling effective P4 medicine, by defining an optimal balance between usability, scalability and data protection, and deploying an appropriate set of computing tools to make it happen. The project aims at creating a platform composed of software packages that seamlessly enable clinical and genomic data sharing and exploitation across a federation of medical institutions, hospitals and research laboratories across Switzerland in a scalable, secure, responsible and privacy-conscious way, and that integrates widespread cohort exploration tools such as i2b2, TranSMART or SHRINE (see Fig. 1). This main goal materializes in the following outcomes that the project will deliver:
– A holistic requirements analysis of the medical data sharing ecosystem, from the standpoint of legal, ethical and medical stakeholders, including a roadmap for progressively addressing these requirements in the clinical and research practice. Aligned with this analysis, the project will keep liaisons with other PHRT/SPHN projects running in parallel, considering their scenarios and accounting for them in the developed security and privacy framework.
– Software-based solutions for accountable and privacy-preserving data sharing featuring trust distribution across a federation of sites with no single points of failure, leveraging existing prototypes such as UnLynx and ByzCoin, and improving on their performance by means of novel approaches based on lattice homomorphic encryption, secure multiparty computation, distributed ledger technologies (a.k.a. blockchains), and distributed access control systems. These solutions will incorporate and adapt widespread tools for cohort exploration and data analysis such as i2b2, TranSMART and SHRINE, and will be prototyped and validated in a real-size scenario.
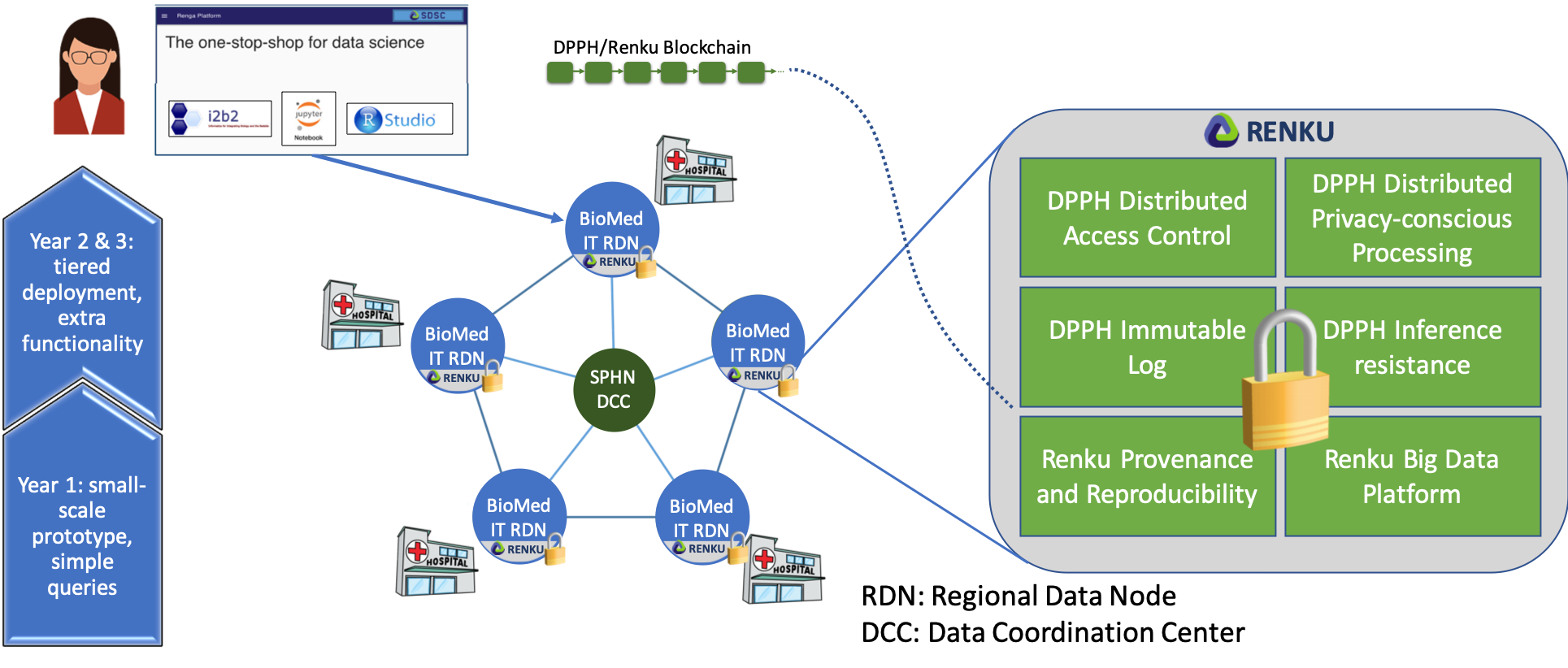
– A quantitative analysis of inference risks, and countermeasures for addressing them when releasing aggregated results on patient data, enabling decision-support systems in medical and *omics scenarios. The patient dimension will also be accounted for by exploring the privacy and security risks of mHealth technologies, by proposing and evaluating solutions aimed at avoiding that pervasive apps can discover the usage of a health-related sensitive app in patients’ devices.
– A comprehensive ethical analysis of distributed platforms for medical data sharing from a normative point of view and through qualitative research.
The first milestone of the project comprises the outcomes of the requirements analysis after the first six months, which will drive an agile development methodology to produce an early prototype that will be deployed at the first year (second main milestone), followed by the development and integration of the aforementioned privacy, security and scalability solutions into a fully functional prototype (third milestone), which will be deployed and validated by the end of the project (fourth and final milestone) in real and practical use cases aligned with the liaised projects.
By bringing together the extensive expertise of the involved groups in genomic privacy and protection of medical data (Hubaux), secure distributed systems (Ford), big-data and knowledge management (Verscheure), cryptographic techniques for decentralized machine learning (Jetchev), ethics of biomedical research (Vayena) and genomic research and precision medicine (Fellay), this project seeks to combine knowledge from the data science, computer science, ethics, medicine and genomics communities to effectively tackle the challenges currently thwarting data sharing for P4 medicine. By establishing liaisons with other PHRT/SPHN projects, DPPH seeks to cover the Swiss national level, targeting prototypes at a national scale, and by leveraging on already established connections with the Global Alliance for Genomics and Health (GA4GH) and its Software Security Group, DPPH also guarantees international relevance and consistency, strengthening the impact of the project.
In summary, this project will produce innovative technical solutions to many privacy-related issues in close collaboration with clinicians, medical researchers and hospital IT specialists in various clinical settings, and will provide the personalized health community with optimal resources to perform cutting-edge research in an ethical and privacy-conscious way.
